
In this half-life problem, we already have a half-life, time, and initial quantity of a radioactive isotope. How many grams of an isotope will remain in 30 years if the half-life of 500 grams of a radioactive isotope is 6 years? Our calculator will simplify the whole process for you.Ĭheck out a few more calculators by us, designed specifically for you. If you don’t want to get yourself into these complex calculations, just put the values in the above calculator. Similarly, you can also calculate other parameters such as initial quantity, remaining quantity, and time by using the above equations. So, if an element with the initial value of 200 grams decayed to 50 grams in 120 seconds, its half-life will 60 seconds.

#Half life chemistry calculator how to#
The foregoing are all three equations that characterize the radioactivity of material and are linked to each other, which can be expressed as follow: How to calculate half life?Īs of now, you have been through the formula for half-life, and you may be wondering how to find half-life by using that half-life equation. Calculating half-life is somewhat complicated, but we will simplify the process for your understanding. The average time a nucleus has remained unchanged. Τ refers to the mean lifetime of an element. Λ refers to the decay constant, which is the rate of decay of an element. Half life formulaīy using the following decay formula, the number of unstable nuclei in a radioactive element left after t can be calculated: However, it is a highly accurate estimate when enough nuclei are available in a radioactive element. It doesn't mean that half of the radioactive element would have decayed after the half-life is over. Half-life is more like a probability measure. For instance, the biological half-life of metabolites.

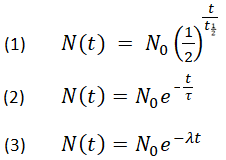
The concept if half-life can also be used to characterize some exponential decay. This shows the variation in the half-life of different elements. Uranium-233 has a half-life of about 160000 years, on the other hand.

The half-life of carbon-10, for example, is only 19 seconds, so it is impossible to find this isotope in nature. Half-life is defined as the time needed to undergo its decay process for half of the unstable nuclei.Įach radioactive element has a different half life decay time. Unstable nuclei are radioactive decay and emit alpha, beta, or gamma-rays that eventually decay to stable nuclei while stable nuclei of a radioactive don’t change. There are stable and unstable nuclei in each radioactive element. Half-life is a concept widely used in chemistry, physics, biology, and pharmacology. It can be used to calculate the half-life of a radioactive element, the time elapsed, initial quantity, and remaining quantity of an element. The Half-Life calculator can be used to understand the radioactive decay principles.


 0 kommentar(er)
0 kommentar(er)
